Resources
Articles in support of Corsano’s scientific approach.Pharma Vigilance
Pharmacovigilance, often referred to as "pharma vigilance," involves the activities related to the detection, assessment, understanding, and prevention of drug-related adverse effects.
PPG Accuracy
The accuracy of Corsano CardioWatch 287 with pulse detection using photoplethysmography technology in cardiac patients was studied by S. Blok [1,2], M.A. Piek [1], I.I. Tulevski [1], G.A. Somsen [1], M.M. Winter [1,3]. In this single-centre prospective study, patients from an outpatient cardiology clinic underwent a simultaneous resting ECG and PPG recording.
Cuffless Non-Invasive Blood Pressure
High blood pressure (hypertension) is a common condition in which the long-term force of the blood against the artery walls is high enough that it will eventually cause heart disease with a patient population of 300m+ across the EU and US. Long-term high blood pressure is a major risk factor for stroke, coronary artery disease, heart...
Sepsis Detection
Sepsis is a life-threatening condition characterized by a dysregulated immune response to infection, leading to organ dysfunction. It is considered a global health concern and a leading cause of death worldwide. Early detection and timely treatment are crucial for improving patient outcomes.
Atrial Fibrillation and Stroke
The most common cardiac arrhythmia is atrial fibrillation (AF). One in four will get it in the course of their life, the probability increases especially from the age of 65, as well as from 55 years with the presence of other risk factors (e.g. high blood pressure, diabetes, previous cardiovascular events) [1].
Cardiac Telemetry
Telemetry rhythm surveillance is standard of care for a large subgroup of hospitalized cardiac patients. Indications for telemetry vary throughout spectra of risks, from direct detection of a life threatening arrhythmia, to determination of a rhythm or conduction disorder, to observation of heart frequency when tailoring treatment.
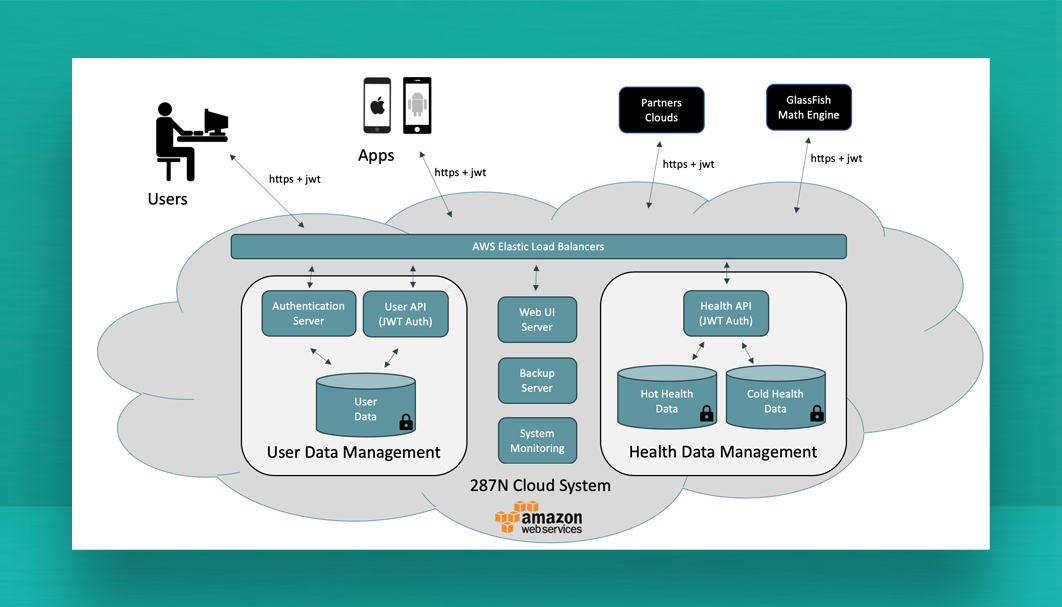
Certified Software
Corsano has medically certified it’s new software platform under ISO 13485 and EU-MDR. Latest technologies have been applied and the Software Development Process has been meticulously documented for CE-MDR and FDA Certification.