Research
Collecting and analyzing data, leading to a better understanding.Research
Corsano’s CardioWatch Bracelet enables the collection of richer data and insights to enhance understanding of the effects of treatment and objective measures of intervention effects during clinical trials.
Incorporating Corsano’s CardioWatch 287 into clinical trial designs has the potential to address many clinical trial challenges, including patient retention and engagement.

Remote patient monitoring makes it easier for patients to participate and improves recruitment through greater access to diverse patient populations.
The use and management of data from CardioWatch offers effective clinical care while protecting the patient with ongoing monitoring, ensuring academic researchers and subjects remain connected wherever they are located.
Corsano’s CardioWatch real-time data also helps researchers detect early signs of adverse events or other issues related to the study, allowing for early intervention and improved patient safety.
By providing raw data, CardioWatch can generate a rich stream of data that can be used to gain insights into patient behavior and vital signs. This data can be made available for AI and machine learning, allowing for more advanced analysis and personalized healthcare.
Corsano is involved in over 30 mostly cardiovascular and oncology clinical trials, examples include:
Supporting Clinical Trials
Clinical trial applications are a core competence of Corsano. It is in use for medical device validations in remote study settings, medical research and the validation of treatments in hospitals.
Corsano developed a Research Portal for physicians and researchers. It connects researchers with patients in a way that makes care convenient and accessible from anywhere. Built on our cloud-based network, Corsano’s application creates a seamless experience across web, mobile, or desktop.
The application enables administration of wearables and data before, during and after clinical trials. It provides workflows for study nurses to onboard patients, manage the device fleet and monitor the status of devices, gateways, data streams and patient compliance.

Incoming data is automatically assessed regarding quality and plausibility. Thereafter, it is reliably stored including metadata like the participant ID, sensor hardware, sensor settings and firmware version. Other metadata can be defined as required. An immutable log trail of system events ensures transparency and auditability.
Processing pipelines allow you to deploy your own algorithms to analyze and transform data streams. Alternatively, you can access or export the stored data via API to analyze it with your tool of choice.

Clinical Apps
Corsano developed various custom applications that enable easy data acquisition with Corsano’s medical-grade bracelets in hospitals and research centers.
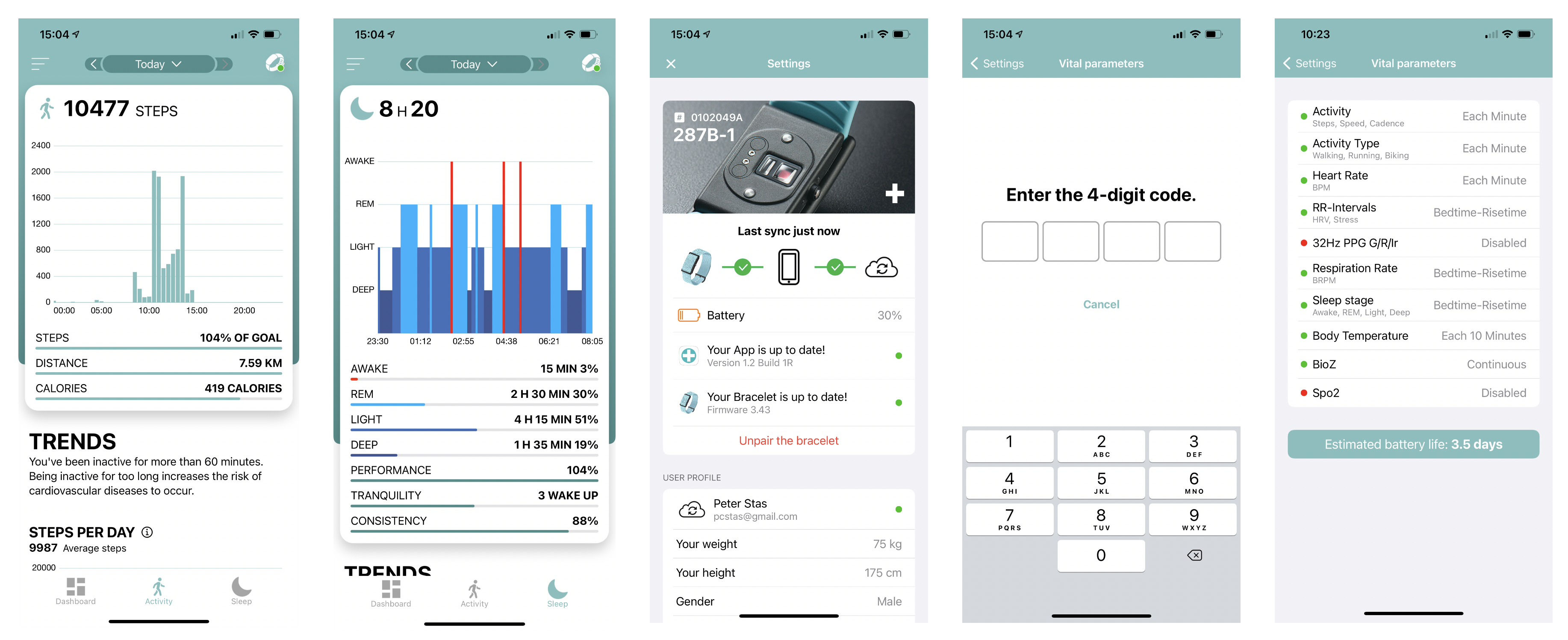
Granular Flexibility
Corsano provides researchers to set which Vital Parameters should be monitored and at which frequency. Corsano offers access to all data, i.e. Raw PPG Green, Red and iR data can be exported up to 128Hz.

Clinical Trials
Corsano CardioWatch is a wrist worn medical grade multi sensor bracelet to capture up to 19 vital parameters. Primary use cases are 24/7 continuous clinical trials and remote patient monitoring.
Research Example Phenotypes in Chronic Heart Failure
An example is Corsano's involvement in a major study to identify congestion phenotypes in patients with chronic heart failure at Duke Heart Center. CardioWatch 287-2 will measure PPG G/R/iR on patients adjunct to an elective right heart catheterization while performing a 6 minutes exercise. A-Line measurements are the gold standard for reference.
Congestion is a hallmark of heart failure (HF). New evidence suggests that volume overload due to salt and water retention is responsible for only half of cardiac decompensations. Inappropriate redistribution of intra-vascular volume might be an additional important driver of cardiac decompensation and can explain an increase in central filling pressures in the absence of weight gain or total body volume increase. There is an urgent need to uncover different congestion phenotypes as this is the basis of personalized (decongestive) HF therapies.
The study at Duke will explore different congestion phenotypes, which will help identify responders to splanchnic nerve block therapy. The overall objective is to perform a comprehensive analysis of the congestion phenotype in patients with chronic HF.

Aim 1: Study the congestion phenotype of chronic HF patients (N=1000) using a variety of traditional and novel congestion measures.
Aim 2: Correlate the non-invasive markers of intra-vascular and extra-vascular congestion with the gold- standard of invasive hemodynamics (RHC)
Interested in conducting a research study with us?
Please tell us more so we can serve you better.