About Us
Certified MedTech companyCorsano Health B.V. is a leading MedTech company that creates, manufactures, and sells advanced real-time remote patient monitoring solutions. Corsano’s offers a CE-MDR medically certified system and FDA Cleared, enabled by the world’s most advanced multi-sensor bracelet for continuous monitoring of vital signs. Unrestricted access to raw data enables AI development to develop life-saving therapies faster. Corsano helps lower overall healthcare costs, generates unparalleled insights, improves clinical outcomes, and enhances the patient experience.
Our History
In the heart of Geneva, entrepreneurs Peter & Aletta Stas laid the foundation for their global watch company, Frederique Constant. From the outset, their entrepreneurial spirit was intertwined with a commitment to societal well-being. Their passion found purpose as they actively engaged with charitable organizations dedicated to combating heart disease.
The turning point arrived when the acquiring entity of Frederique Constant expressed disinterest in the Smartwatch division. Peter and Aletta seized the opportunity to spin this division off into a separate company to develop medical technology. Thus, Corsano Health emerged in the field of continuous patient monitoring with wearables to advance healthcare. The Corsano brandname is derived from in corpore sano (in a healthy body).
Fast forward six years, and the Corsano CardioWatch System has been utilized in over 100 rigorous clinical studies. Groundbreaking research is currently being conducted in therapeutic fields such as Arrhythmias, Hypertension, and Immune Oncology using the system. For Peter and Aletta, their dream of making a transformative impact on healthcare has transcended aspiration to drive the company.
At Corsano Health, our narrative is not just about technology; it's about a profound commitment to advance healthcare through continuous patient monitoring. Join us on this extraordinary journey as we continue to redefine the boundaries of healthcare excellence.
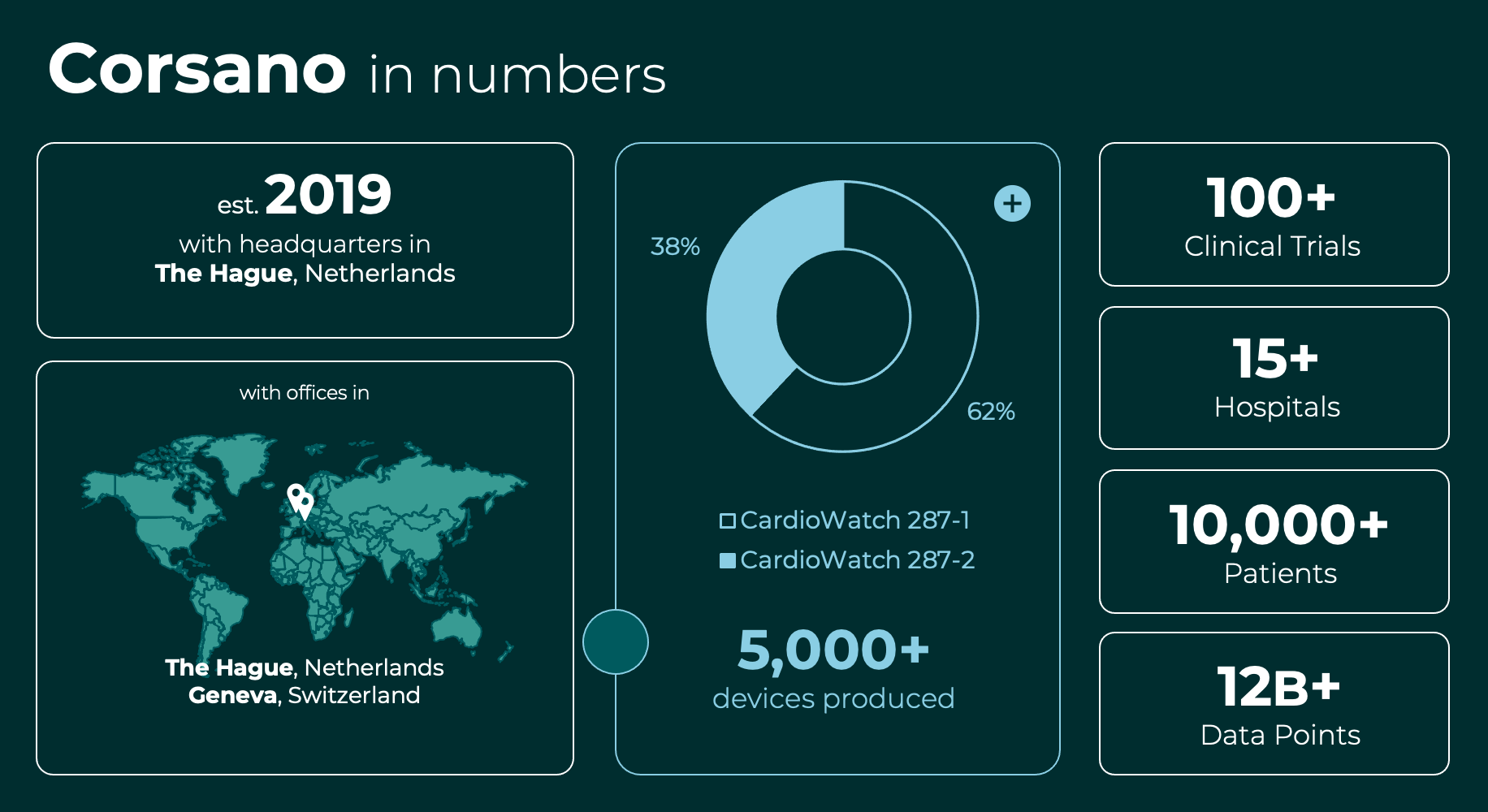
Note: 100+ Clinical Trials includes completed, on-going and scheduled trials with the CardioWatch System by Corsano and third parties. Please contact us for more information.
Corsano Health B.V. achieved certification as a medical device manufacturer according to the ISO 13485 norm under the new MDR regulations in November 2021. In addition, the first product CardioWatch 287 achieved CE certification as a medical product under the new MDR regulation. Clinical accuracy is paramount for us. In addition to third parties’ algorithms, we run our proprietary algorithms to realize superior data accuracy. Corsano technology has been clinically validated in major academic hospitals. The bracelet is manufactured under ISO 13485 and CE-MDR compliance. FDA cleared the Corsano CardioWatch 287-2 System.
Dutch Heart Foundation awarded Corsano and RadboudMC a ~€1m development project to recognize cardiac arrest and alert emergency services (out of 15 parties). Corsano has been developing a certified medical solution that can be sold in Europe and the USA to over 1M patients (includes device and subscription services). Clinical trials started in 2nd half 2022 with up to seven academic hospitals acquiring evidence to apply for CE-MDR and FDA Clearance.
What Differentiates Corsano Health

Device and platform EU-MDR certified, FDA 510(k) Cleared. Validated and proven with clinical trial data. QMS enables to add sensors, algorithms to Medical platform. +4 years head start.

35 years experience, ergonomic product, choice of materials, craftsmanship, battery life of 8-15 days, ergonomic and aesthetic designs that stimulate continuous wear.
Supply chain wearables, deep relationships with suppliers, Swiss quality, agility to rapidly develop next generations.
The CardioWatch monitor offers flexibility to set data collection intervals by minute, by second or 25Hz, 32Hz and 128Hz. We measure Heart Rate, RR intervals, Breathing Rate, A-Fib detection, SpO2, Core Body Temperature, ECG, Blood Pressure, Activity and Sleep.
