Corsano Cuffless Non-Invasive Blood Pressure
Collaborating researchers from Reinier de Graaf Hospital have validated the Corsano cuffless photoplethysmography-based wristband for measuring blood pressure according to the regulatory standards. This was demonstrated in 97 patients wearing Corsano CardioWatch 287-2 while undergoing cardiac catheterization. These results were published in the scientific journal The European Heart Journal.

The following graphical abstract describes the research.

Background
Blood pressure (BP) monitoring is essential in clinical and ambulatory care settings for accurate diagnosis and management of various medical conditions. However, current BP measurement methods encounter challenges, including the fact that it is time consuming, along with record-keeping difficulties. The gold-standard for BP measurement is intra-arterial measurement, however, the use of uncomfortable indwelling catheters poses risks to vulnerable patients. Hospital cuff BP measurement can be influenced by confounding factors like isolated office hypertension and masked hypertension. Additionally, the principle of vessel compression and subsequent blood flow alterations used in cuff BP measurements does not correlate well with invasive measurements in conditions such as high BP or widespread vessel atherosclerosis. In ambulatory care, the sporadic nature of measurements may fail to capture true BP patterns over time, hindering optimal management of high BP.
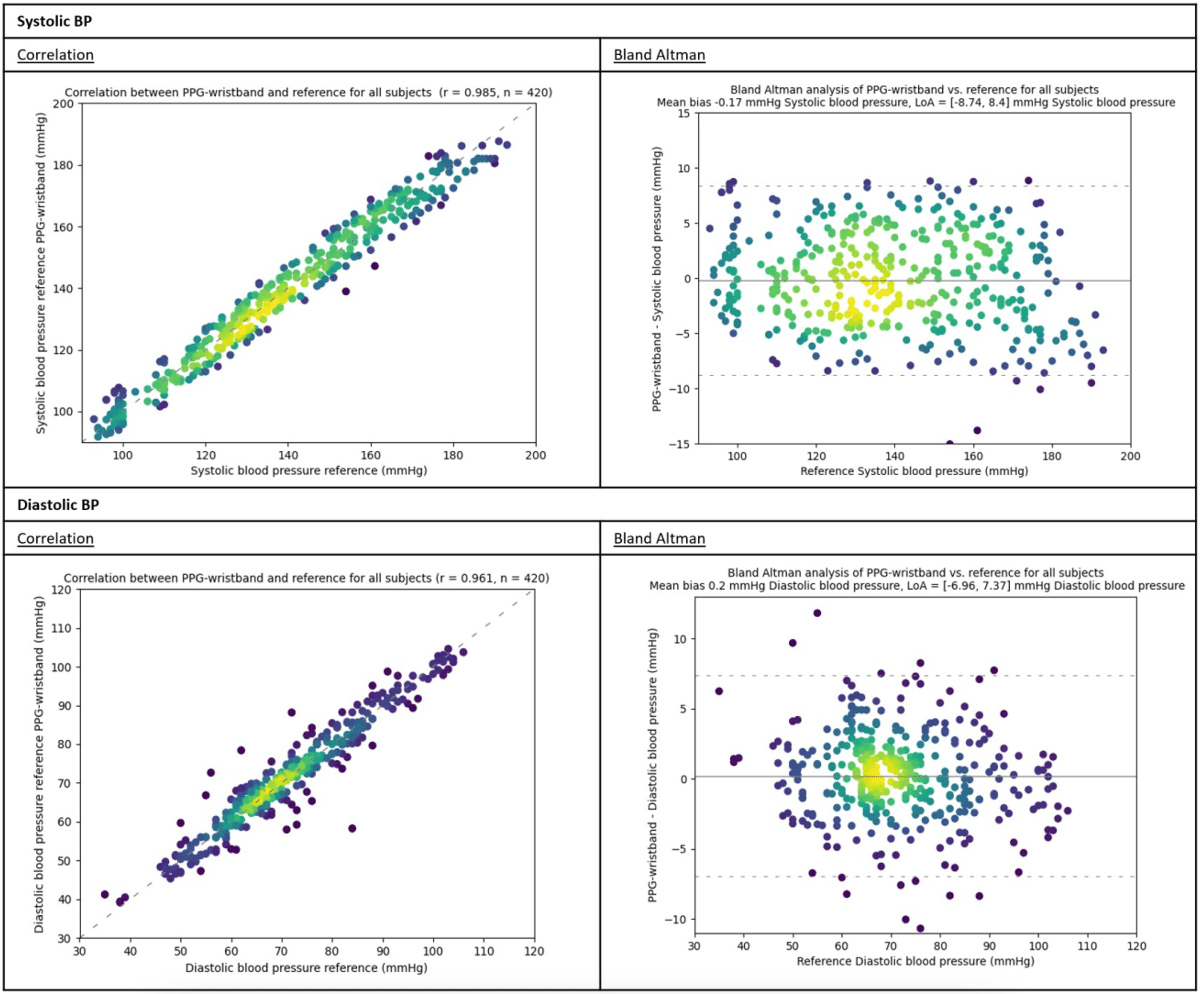
Results

Conclusion
This study provides evidence for the accuracy of the combination of the CardioWatch 287-2 PPG-based wristband and its BP-algorithm against current EU regulatory standards, demonstrating the feasibility of this technique for BP monitoring. The continuous monitoring capabilities, coupled with the potential for real-time analysis and early disease detection, hold great promise for improving patient care and outcomes. Further research is ongoing to address the limitations of this study and evaluate the performance of the PPG-based wristband in light of the remaining ESH recommendations as well as in an ambulatory setting.