Title
SmartAF: Monitoring of Atrial Fibrillation in Hospitalized Patients for Stroke Prevention
Topic
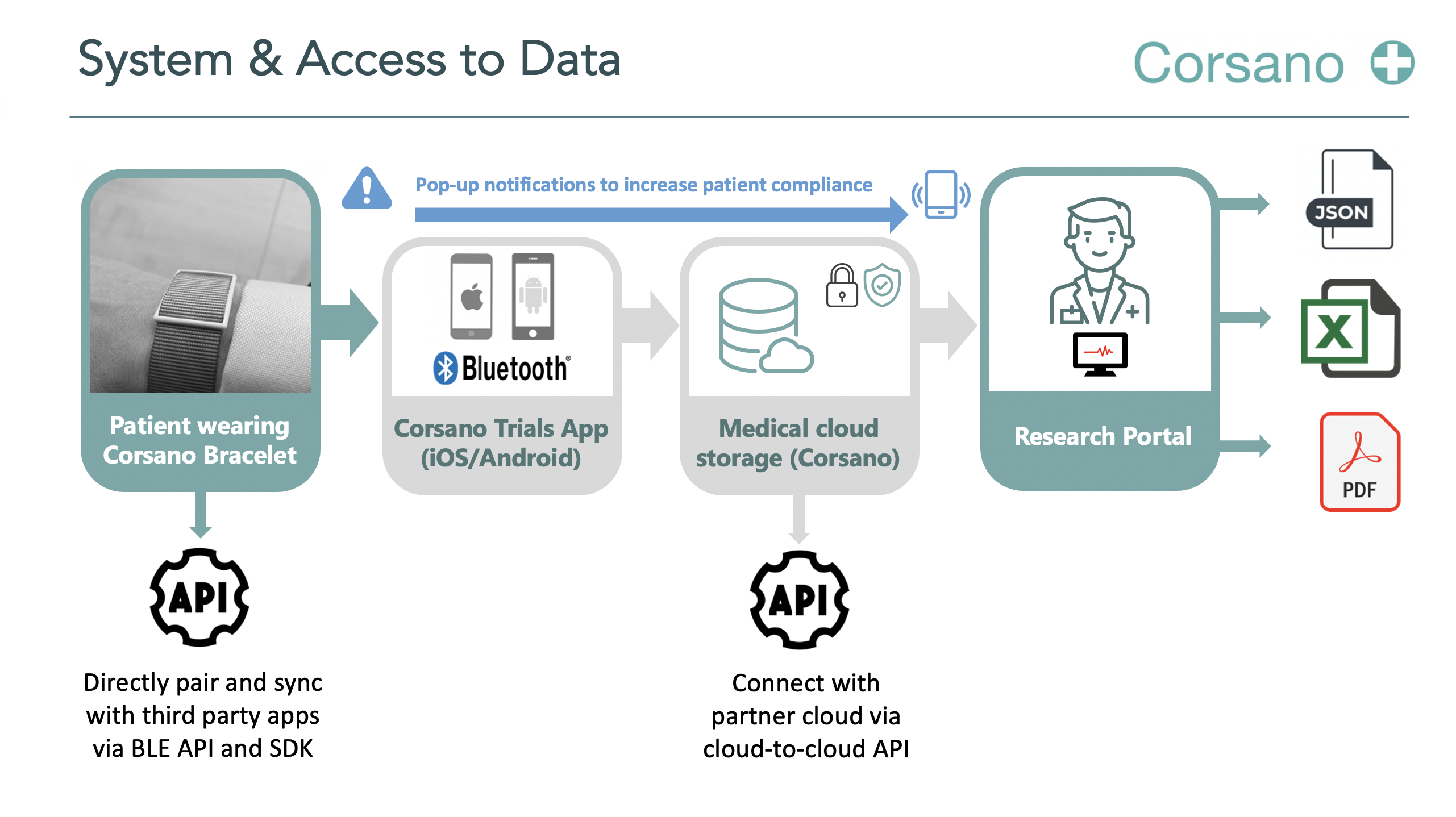
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major risk factor for cerebrovascular insults. Screening for AF in hospitalized patients is challenging. The classical ECG monitoring requires the patient to wear patch electrodes and cables, which is uncomfortable and may lead to skin irritation and reduced mobility. In this trial the Corsano CardioWatch 287 will be used to conduct continuous heart rate and –rhythm monitoring in hospitalized patients with moderate to high risk for AF. Collected data will then be automatically de-identified and send to Preventicus for analysis. The algorithm is a clinically validated, CE marked and certified medical device (class IIa). If an arrhythmia is detected, the patients will receive a 7d Holter ecg for confirmation.
Number of Participants
250 hospitalized patients
Inclusion / Exclusion criteria
Inclusion criteria
Hospitalized patients on the internal medicine ward of USB
CHA₂DS₂-VASc Score ≥ 2
Written informed consent as documented by signature from the patient
Exclusion criteria
Current or prior diagnosis of AF
Chronic anticoagulation therapy for other reasons
Cardiac implanted electronic device (Pacemaker, ICD)
Smartwatch cannot be worn due to comprehensible reasons (allergic reactions, wounds, amputations, other)
Unable or not willing to sign informed consent
Study Design
This is an investigator-initiated, prospective, single-center observational trial to evaluate the performance and accuracy of a diagnostic algorithm provided by Preventicus in identifying hospitalized patients with atrial fibrillation. 250 patients hospitalized in the internal medicine department will be enrolled in this study. Patients without prior history of AF and increased CHA₂DS₂-VASc Score (≥ 2) will be approached and will receive a wearable device provided by MMT for PPG-based continuous heart rate and –rhythm monitoring. Data will be transmitted to a local server via the USB ICT-platform, de-identified and forwarded to Preventicus for analysis. Preventicus will analyse the data files and forward it for a second quality test to Telecare Ulm if an arrhythmia is suspected. If the quality check is positive the likelihood of AF is >90%. In this case the report will be send back to the USB and re-identified. The Sponsor-Investigator will be informed and the respective patient will receive a 14-day Holter ECG. If AF is confirmed, subsequent consultation with a cardiologist will be recommended.
Target points
The primary outcome will be
Detected AF episodes
The secondary outcomes include:
AF screening durations
Health economic simulation: costs per identified AF case after AF screening and after AF confirmation
Study Centre
University Hospital Basel
Start time, Duration
Start: september 2021,1.5 years
Interested in our Trial Programme?
Corsano Cardiowatch Bracelets enable continuous monitoring with multiple algorithms. Corsano is working closely with cardiologists, scientists, hospitals, patients, and research organisations. Scientific research demonstrates the legitimacy of Cardiowatch 287 algorithms.
We are currently performing pilots with selected clients. Contact us if you want to know more!
The founders of Corsano Health have over 100 years of experience in the Swiss Watch industry, with deep experience about ergonomic design and materials for wearables that are worn 24/7.