Title
Case-finding Study to Detect and Quantify Episodes of Absolute Arrhythmia Using an Automated, Wearable Monitoring System (Smart in OAC)
Topic
To demonstrate that patients with previously unknown atrial arrhythmias can be detected by a wearable in combination with Preventicus Heartbeats Core using a completely digital patient data capture system.
Number of Participants
Patients to be screened: 1,000
Inclusion criteria
• Informed consent
• Mobile phone compatible with the PPG-wearable (see Appendix 3)
• Stable internet connection
Exclusion criteria
• Unwillingness to participate
• Inability to consent
• Known AF
• Known OAC treatment
Study Design
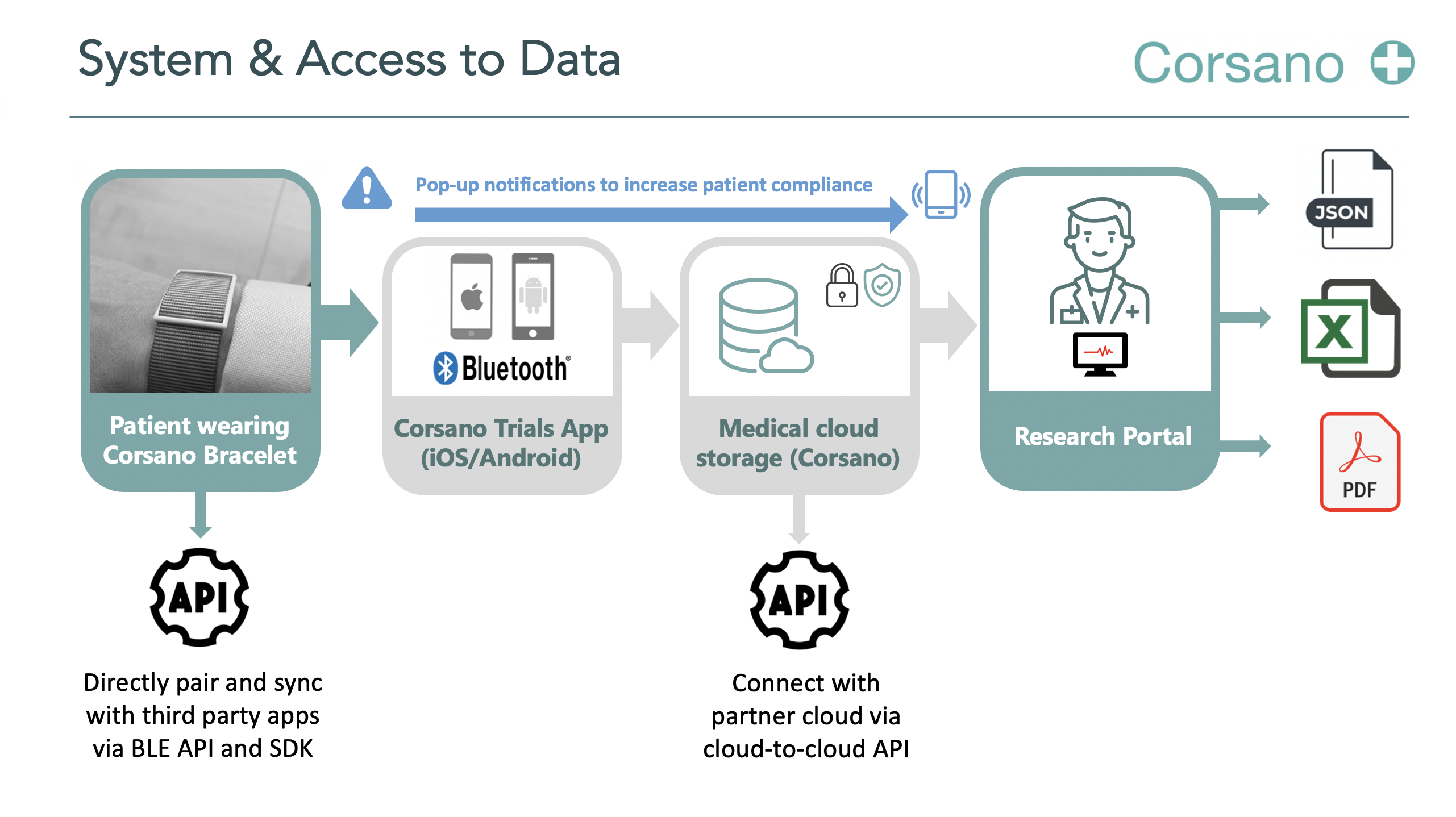
In this study, the feasibility of evaluating the efficacy of a cloud based analytic service in combination with a Corsano CardioWatch 287 in detecting AA will be assessed and the number of cases found. The design aims to provide simple, low-threshold access to this screening technology targeting at-risk populations. This study will not cause any restrictions on the usual care of the study participants. Access to the screening will be provided close to home and free of charge. The app will also be used to validate and enhance the clinical information about the participants captured during the study. This information will be used to define and refine target groups with highest screening efficiency and, in the long-term, outcome benefits. The study will describe the prevalence of AA in an unselected population that can be reached by a low-threshold screening procedure. The study will also generate important information on the different possible screening environments in different countries (e.g. pharmacies, GP practices, etc). Structures of work-up and continuous patient management in screen-positive individuals will be described and may help to design screening pathways in the main trial. By verifying the wearable-diagnoses by ECG in all screen-positive and a random selection of screen-negative participants, the diagnostic accuracy of the wearable in combination of cloud based analytic service can be estimated. Cost effectiveness assessment will evaluate the cost of low-threshold remote screening per patient identified and help guide to target high risk groups with optimal screening yield in the future. The collected data will provide the sound basis for the design and conduct of a large outcome trial.
Target points
The primary outcome will be
- Number of participants reached
- Number of participants with detected absolute (atrial) arrhythmia.
The secondary outcomes include:
- Rate of participants with newly detected absolute (atrial) arrhythmia
- Rate of participants with confirmed absolute (atrial) arrhythmia (sub-analysis: atrial fibrillation)
Measurement procedures
Reference to evaluate the performance and accuracy of the Preventicus Heartbeats algorithm are the diagnosis of the 14d Holter ecg, analysed by cardiologists.
Study Centre
Germany: Hamburg
UK: Birmingham
Spain: Barcelona
Poland: Krakow (or Katowice)
Start time, Duration
Start: Q1 2021, Total study duration: 12 months
Duration per patient: 2 months
Interested in our Trial Programme?
Corsano Cardiowatch Bracelets enable continuous monitoring with multiple algorithms. Corsano is working closely with cardiologists, scientists, hospitals, patients, and research organisations. Scientific research demonstrates the legitimacy of Cardiowatch 287 algorithms.
We are currently performing pilots with selected clients. Contact us if you want to know more!
The founders of Corsano Health have over 100 years of experience in the Swiss Watch industry, with deep experience about ergonomic design and materials for wearables that are worn 24/7.