Title
A study evaluating the safety and efficacy of anti-CD19 CAR T in subjects with Lymphocytic Lymphoma.
Topic
A phase I/II, multicenter study evaluating the feasibility, safety, and efficacy of point-of-care manufactured 19CP02 in subjects with relapsed/refractory B-cell non-Hodgkin lymphoma.
Number of Participants
50 subjects
Study Design
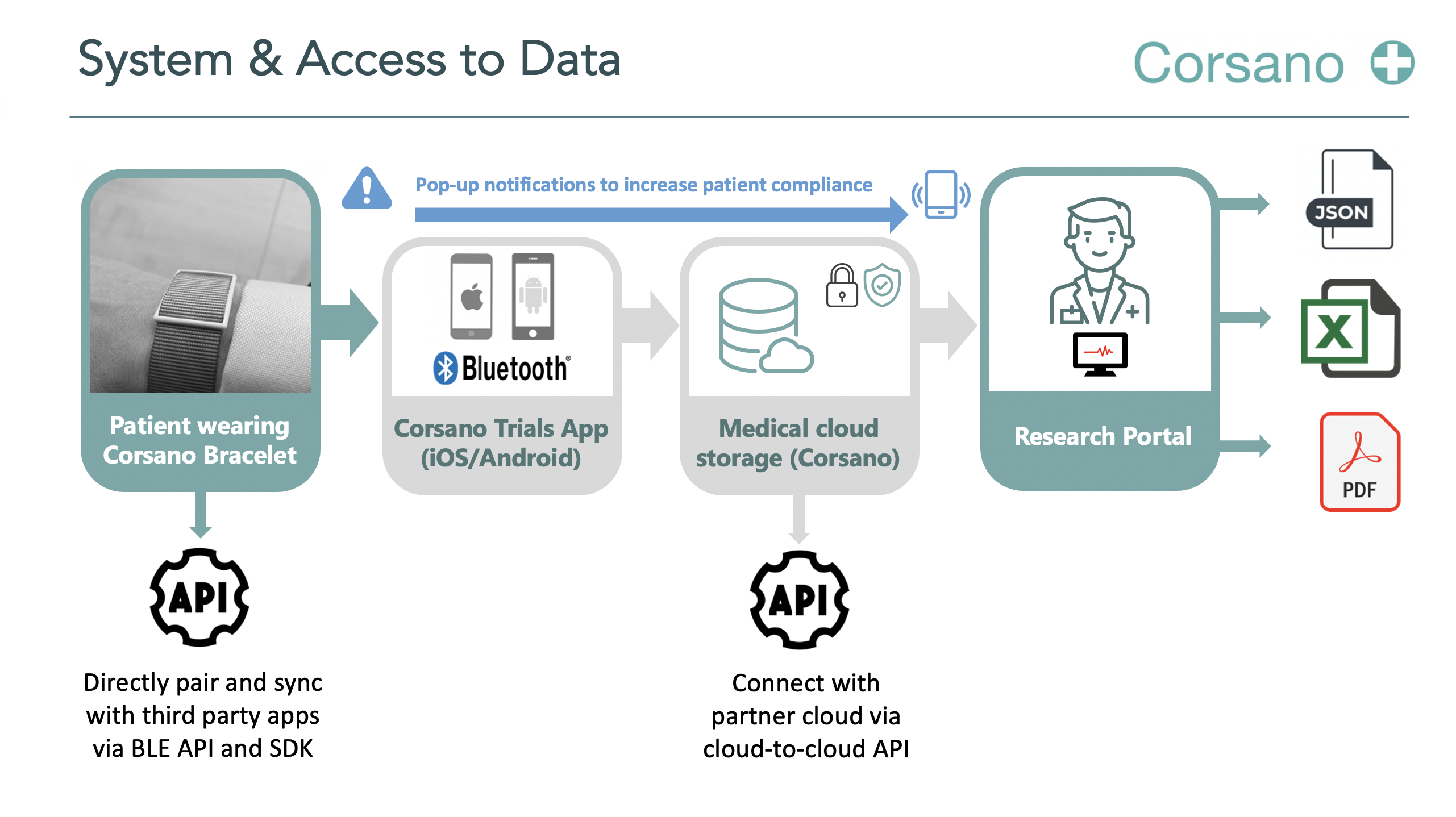
Corsano will deliver CardioWatch 287-2 System to interact with trial patients and to simplify the way patient data is collected and processed (answers to questions, vital signs, etc.). The App will facilitate interaction with patients.
Inclusion Criteria
1. Signed informed consent form
2. Age ≥ 18 years
3. Histologically confirmed diagnosis of one of the following non-Hodgkin lymphoma subtypes: DLBCL, FL grade 1, 2 or 3A, MZL, or MCL
4. Relapsed or refractory disease
5. Measurable disease according to the Lugano classification
6. ECOG performance status of 0 or 1 (ECOG 2 is allowed if due to underlying disease)
7. Adequate bone marrow, renal, hepatic and pulmonary function
Exclusion Criteria
1. Primary CNS B-cell lymphoma, Burkitt lymphoma, or Richter’s transformation
2. Selected prior treatments as defined in the protocol
3. History of malignancy other than lymphoma (exceptions per protocol)
4. Active CNS involvement (with neurological changes) by disease under study
5. Infection with HIV, hepatitis B or hepatitis C virus
Study Centre
Major Pharma Client (NL)
Start time, Duration
February 2022, 36 months
Interested in our Trial Programme?
Corsano Cardiowatch Bracelets enable continuous monitoring with multiple algorithms. Corsano is working closely with cardiologists, scientists, hospitals, patients, and research organisations. Scientific research demonstrates the legitimacy of Cardiowatch 287 algorithms.
We are currently performing pilots with selected clients. Contact us if you want to know more!
The founders of Corsano Health have over 100 years of experience in the Swiss Watch industry, with deep experience about ergonomic design and materials for wearables that are worn 24/7.