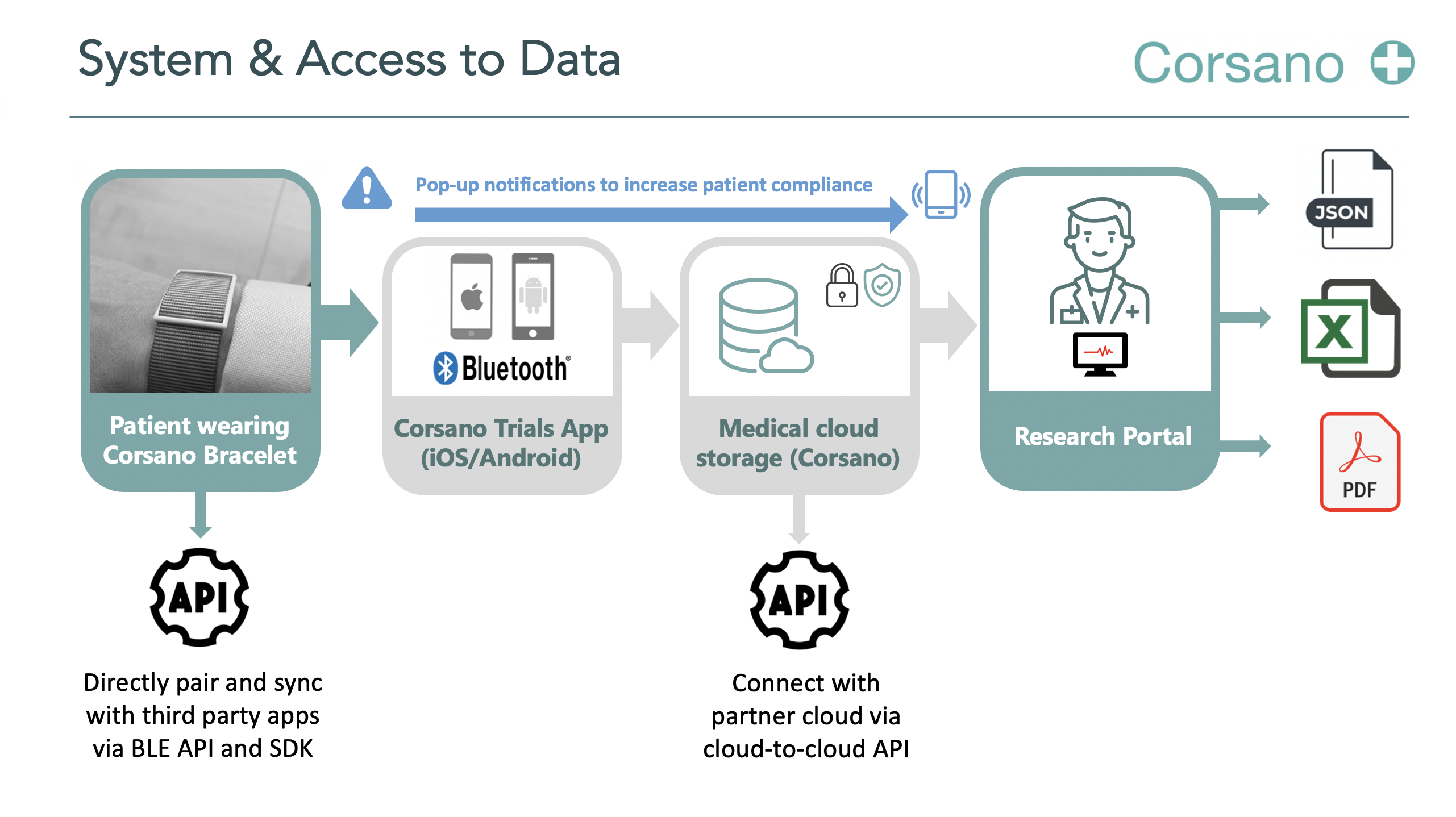
Title
RECAMO: REmote CArdiac MOnitoring by the Corsano Cardiowatch 287-2 of BP and AF
Rationale
Wearables have the potential to monitor patients remotely. The Corsano CardioWatch 287-2 is such a medical device that can monitor atrial fibrillation and long-term blood pressure. The device has been validated using clinical trials in hospitals, but evaluation in the intended remote setting is lacking.
Study design
The study is a single center, single arm prospective study
Primary objective
To compare the number of episodes of atrial fibrillation detected by the Corsano CardioWatch 287-2 during 28 days of use with the number of episodes of atrial fibrillation detected by conventional Holter monitoring during 48 hours of use (standard care).
Secondary objective
To assess the difference in blood pressure measurements obtained by the Corsano CardioWatch 287-2 and the conventional cuff blood pressure monitor over a period of 28 days; to assess the usability of the Corsano CardioWatch 287-2 from a patient perspective.
Study population
One group (A) receiving an EKG monitoring holter for the duration of 24 hours as part of standard care for atrial fibrillation screening. Besides, one group (B) receiving an automatic blood pressure cuff for the duration of 24 hours as part of standard care for blood pressure evaluation.
Main study parameters/endpoints
(1) Absolute percentage increase of patients in whom at least one event of atrial fibrillation is detected by the Corsano CardioWatch 287-2 during 28 days of monitoring, compared to a conventional EKG holter during 24-48 hours of monitoring.
(2) Mean blood pressure difference and its SD between blood pressure measured by the Corsano CardioWatch 287-2 and blood pressure measured by a conventional oscillometric blood pressure cuff.
Secondary study parameters/endpoints
(1) Bias and limits of agreement between blood pressure measured by the Corsano CardioWatch 287-2 and blood pressure measured by a conventional oscillometric blood pressure cuff.
(2) Usability of the Corsano CardioWatch 287-2 in a remote care setting.
Number of Participants
160 patients at the catheterization laboratory of Reinier de Graaf Gasthuis Delft during ABPM or Holter exam, measurements will take place by the Corsano CardioWatch 287-2 and a reference device.
Inclusion / Exclusion criteria
Inclusion criteria:
≥ 18 years old;
undergoing coronary angiography;
able to provide consent
Exclusion criteria:
Unable to wear the Corsano CardioWatch 287 due to reasons such as allergic reactions, wounds, amputations etc.;
Unable to receive blood pressure measurements per cuff due to lymphedema, amputation, dialysis shunt, wounds, etc.;
Pregnant women;
Breastfeading women;
Study Centre
Reinier de Graaf Gasthuis Delft (NL)
Start time, Duration
May 2023, 6 months
https://www.clinicaltrials.gov/study/NCT05899959
Interested in our Trial Programme?
Corsano Cardiowatch Bracelets enable continuous monitoring with multiple algorithms. Corsano is working closely with cardiologists, scientists, hospitals, patients, and research organisations. Scientific research demonstrates the legitimacy of Cardiowatch 287 algorithms.
We are currently performing pilots with selected clients. Contact us if you want to know more!
The founders of Corsano Health have over 100 years of experience in the Swiss Watch industry, with deep experience about ergonomic design and materials for wearables that are worn 24/7.