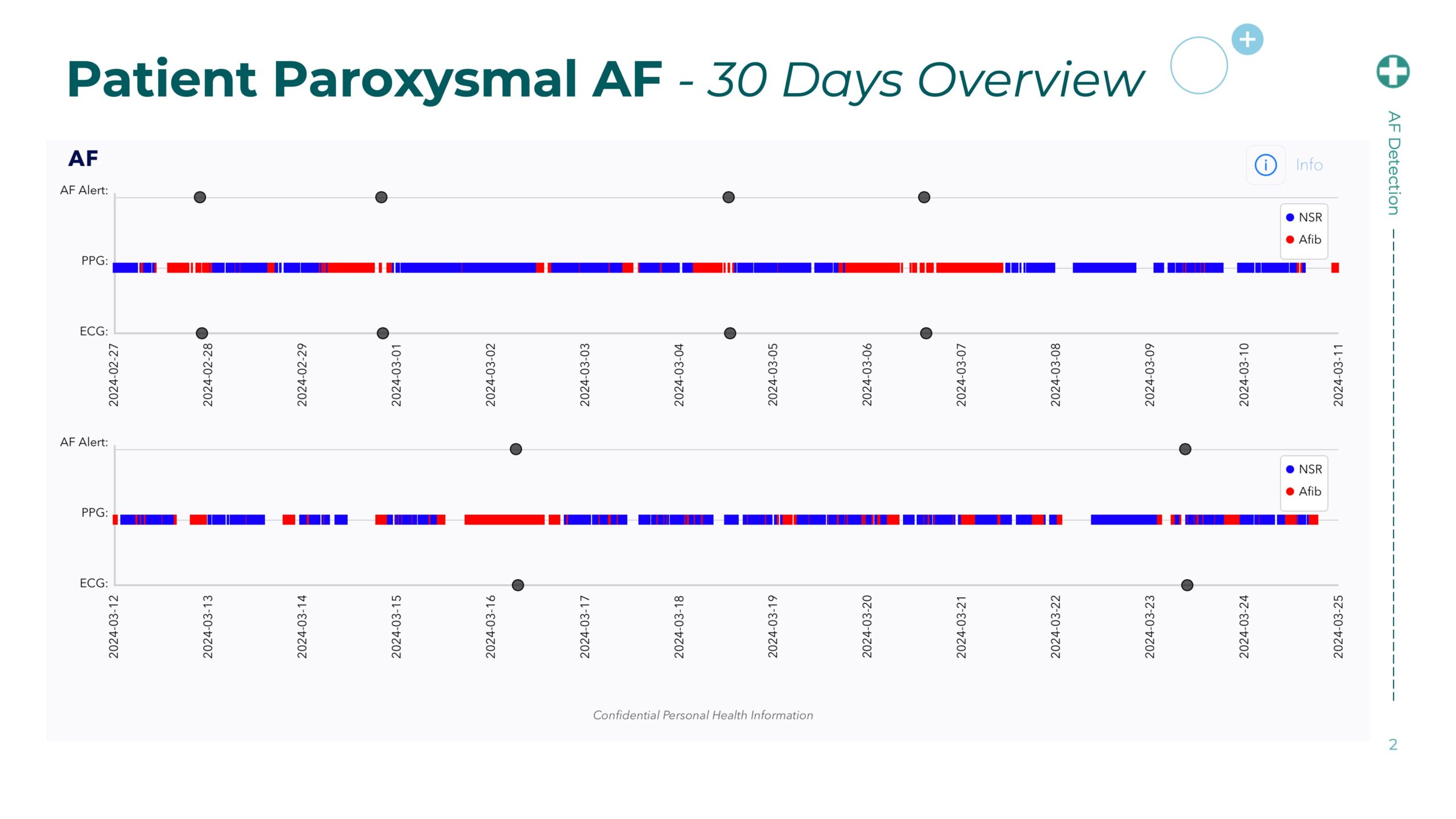
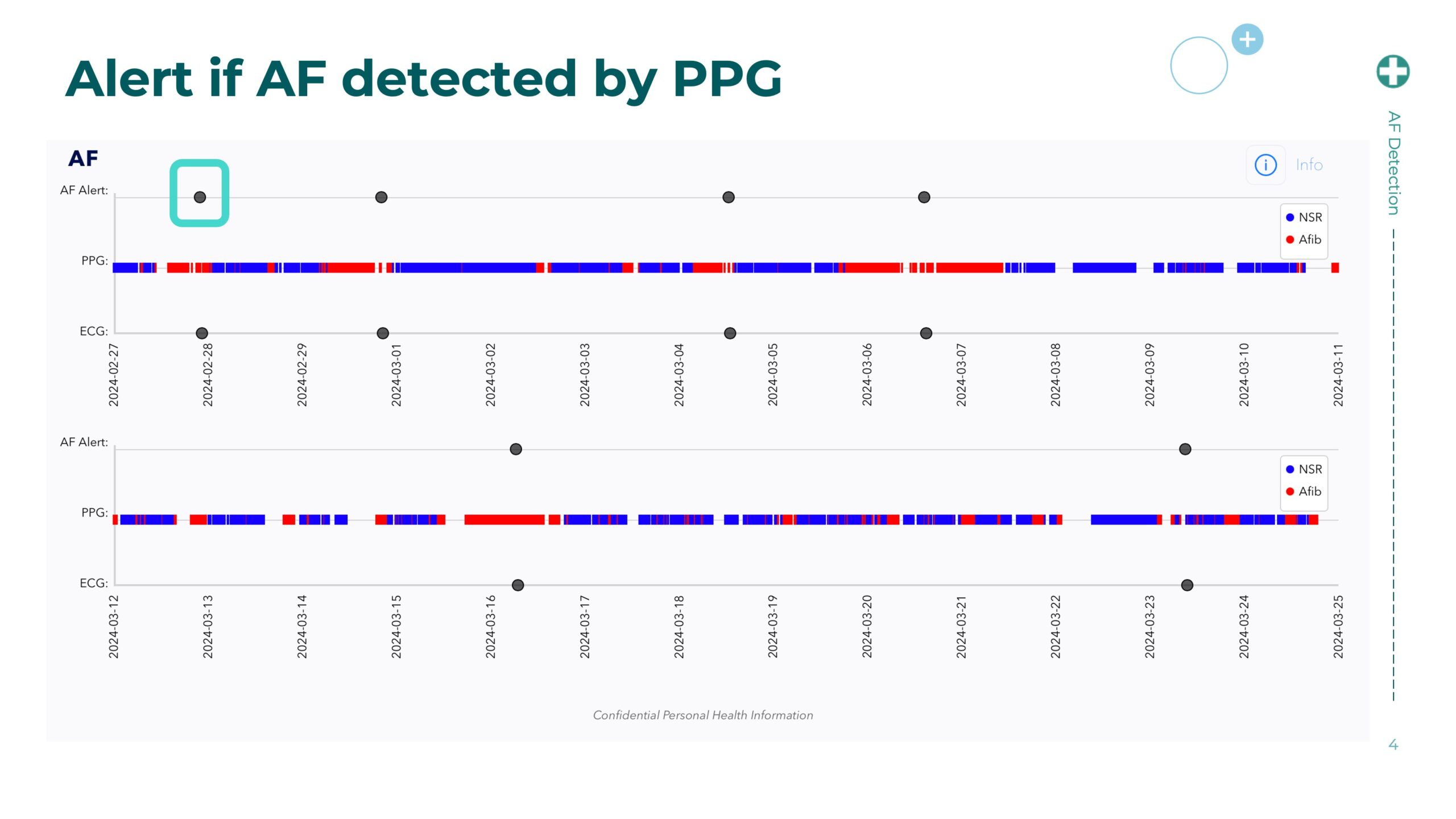
Corsano Health and Cena Research Institute Launch Wearable-Based AFib Detection Study to Transform Atrial Fibrillation Care
The Hague, November 20, 2025: Corsano Health, a leader in next-generation wearable cardiovascular monitoring, has partnered with the Cena Research Institute (CRI) to launch a real-world clinical trial focused on AI-based atrial fibrillation (AFib) detection using the Corsano CardioWatch 287-2 device. The study will be conducted across multiple Houston Cardiology Consultants locations to validate the accuracy, usability, and clinical impact of continuous AFib monitoring in diverse patient populations.

The CardioWatch 287-2 is a medically validated photoplethysmography (PPG)-based wearable designed for continuous, high-fidelity physiological monitoring, including heart rhythm irregularities such as AFib. Through this collaboration, Corsano and CRI aim to accelerate the clinical evaluation of digital health wearables for AFib recognition—especially in patients at risk of stroke or on rhythm-control therapies.
“Early and accurate AFib detection can change lives—but current solutions often miss intermittent events” said Dr. Asif Ali, Director of the Cena Research Institute. “By bringing Corsano’s continuous monitoring technology directly into patients’ daily lives, we’re building a model for real-time AFib management that is practical, scalable, and accessible.”
Study Objectives:
• Validate continuous AFib detection accuracy of the CardioWatch 287-2 against clinical standards
• Assess user engagement and experience with long-term wearable monitoring
• Evaluate the role of wearables in improving anticoagulation adherence and reducing stroke risk
• Support the development of future remote patient monitoring (RPM) and risk stratification pathways
• Generate real-world data to inform FDA submissions and payer adoption
The study will also support CRI’s mission to improve cardiovascular research equity by engaging historically underrepresented patient groups and incorporating digital health literacy and usability assessments.
“Our partnership with Cena represents a key step in scaling real-world cardiovascular monitoring” said Peter Stas, Chief Executive Officer of Corsano Health. “We believe the future of cardiology depends on continuous, patient-centered data and this study brings that vision one step closer to reality.”
Clinical Sites
The trial will enroll patients across multiple clinical locations, including:
8830 Long Point Road, Suite 507, Houston, TX
509 W Tidwell Road, Suite 130, Houston, TX
3535 Briarpark Drive, Suite 101, Houston, TX
An anonymized dataset will be generated from the study to support the development of AI algorithms, event detection models, and next-generation remote monitoring platforms.
About Cena Research Institute
Founded in 2014 and based in Houston, TX, the Cena Research Institute (CRI) is a physician-led research organization dedicated to inclusive, real-world clinical validation in digital health, biotechnology, and medical innovation. CRI partners with global research teams and healthcare systems to bring cutting-edge solutions to underserved communities.
About Corsano Health
Corsano Health provides medically validated wearable devices that deliver continuous, high-quality physiological data for clinical trials, health systems, and patient self-monitoring. The Corsano platform integrates seamlessly into digital health ecosystems, delivering scalable insights for cardiovascular research, remote monitoring, and precision care.